Summary
- BioXcel product candidates use the 505(b)(2) approval pathway.
- Successful pivotal trials for two indications in acute agitation.
- Well funded for operations through 2022.
- Looking for more investing ideas like this one? Get them exclusively at The Total Pharma Tracker. Get started today »
BioXcel Therapeutics, Inc. (NASDAQ:BTAI) is a clinical stage biopharmaceutical company developing drug candidates using artificial intelligence to improve neuroscience and immuno-oncology therapies. On the cusp of its first approval with successful pivotal trials, the company is well-funded with miniscule debt to carry out its operations through 2022.
Pipeline and catalysts
The company has designed and is implementing a clinical development program that takes advantage of the 505(b)(2) New Drug Application (NDA) regulatory pathway, utilizing the existing clinical and safety dataset of intravenous, or IV, formulation of dexmedetomidine (DEX), a selective alpha-2 adrenergic receptor agonist that directly targets the causal mechanism. Lead product candidate, BXCL501, is a sublingual thin film of dex, designed for ease of administration and rapid onset of action.
BioXcel’s development pipeline includes two more indications for BXCL501 - acute agitation in dementia (phase 1b/2), and opioid withdrawal (IND clearance). Another candidate is BXCL701, an orally-available systemic innate immunity activator with dual mechanisms of action - inhibit dipeptidyl peptidase (DPP) 8/9, and block immune evasion by targeting fibroblast activation protein (FAP). BXCL701 is presently being evaluated in phase 2 trials for treatment of neuroendocrine prostate cancer (tNEPC) and for pancreatic cancer in combination with other immuno-oncology agents. The candidate is also being evaluated in combination with KEYTRUDA in an open-label phase 2 basket trial led by MD Anderson for indications with unmet needs in advanced solid tumors.
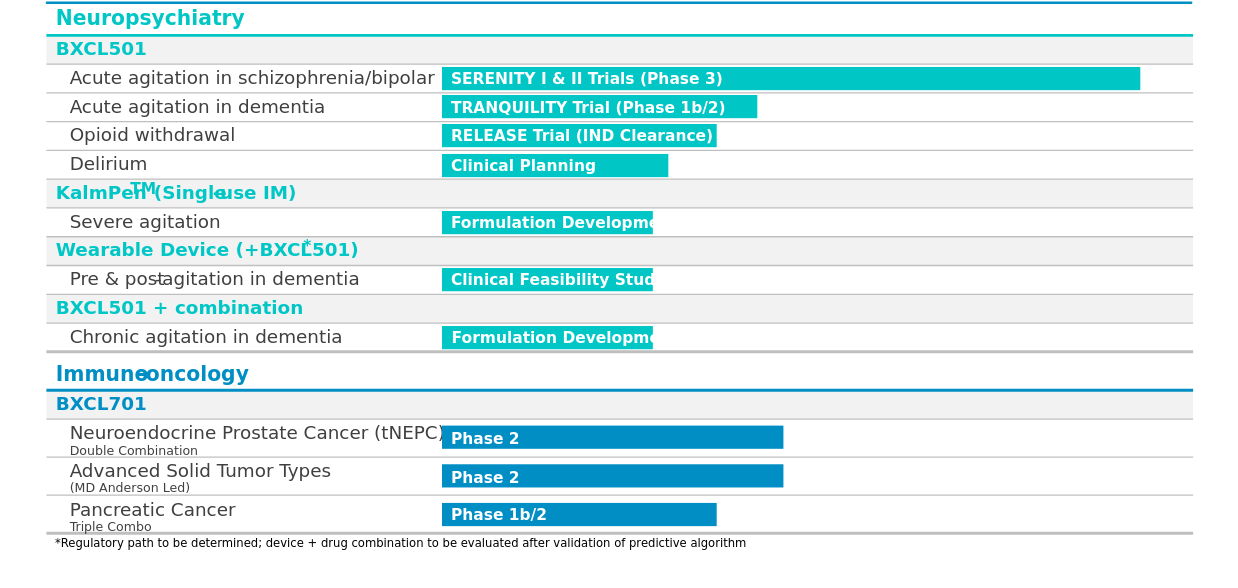
Image source: company website
The company reported positive results in July 2020, from two pivotal SERENITY trials of BXCL501 for the treatment of acute agitation in schizophrenia and bipolar disorder patients. The company is planning to submit NDA for BXCL501 in both indications to the U.S. FDA in the first quarter of 2021, after a pre-NDA meeting with them this month.















